Introduction. At present, around the world there is a significant increase in the incidence of inflammatory bowel disease (IBD) [11,15,16,18,22]. Among IBD cases the ulcerative colitis (UC) is of particular relevance, given the predominant involvement of young people, disease severity and the presence of life-threatening complications [3,7].
UC is characterized by chronic relapsing diffuse inflammation of the colonic mucosa with the development of intestinal and extra-intestinal manifestations and complications [1,4,5,8]. Currently, an integrated diagnostic approach including clinical examination, radiological, endoscopic and histological methodsis used for its diagnostics. One of the modern ways of assessing the state of the mucous membrane of the colon is an immunohistochemical study with the definition of markers of proliferation and apoptosis.
Thus, with monoclonal antibodies, which are cell cycle phase specific, and their subsequent visualization bymeans of immunohistochemistry, we can estimate the process of proliferation and apoptosis in cells [9]. The most commonly used proliferation markers are antigens PCNA and Ki-67. It is known that PCNA (Proliferating Cell Nuclear Antigen) is involved not only in cell proliferation, but also in the repair of DNA after damage [13], which makes the antigen conditionally cell cycle specific, since DNA repair may be carried out in a resting phase [2, 20]. Another antigen, significantly associated with the phases of the cell cycle, is Ki-67. The expression of this protein occurs during phase G1, increases during the cell cycle and decreases sharply in mitosis phase [14]. This protein, unlike PCNA,is not involved in DNA repair [19]. Thus, the expression of Ki-67 makes it possible to identify the cells present in all phases of the cell cycle except the resting phase G0 [12,17]. Among the most studied regulators of apoptosis are the bcl family and protein p53. The p53 protein is unstable, it is localized in the cell nucleus. In case of DNA damage the protein becomes stable, and its concentration in the nucleus increases. There are two types of p53 – the “wild” and the mutant. The first is forming in a normal state, has a very short half-life period and, therefore, can not be defined in tissues by means of immunohistochemistry. The mutant protein can bind up with the “wild” one, fully inactivating it, which may lead to the stimulation of tumor growth. The accumulation of p53 in the cell nucleus initiates apoptosis even in the absence of DNA damage [10, 23]. The p53 gene controls the expression of genes family bcl2 located on intracellular membranes [21], which also regulates the process of apoptosis.
The purpose of the study. To analyze the expression of markers of apoptosis (p53) and proliferation (Ki-67) in patients with ulcerative colitis, depending on the duration and severity of the disease, localization and endoscopic activity of the inflammatory process.
Materials and methods. The study group comprised 80 patients with ulcerative colitis at the age of 18 to 66 years (33 women and 47 men), the comparison group – 15 practically healthy people. The examination of patients was performed using clinical, laboratory, endoscopic and morphological methods, as well as immunohistochemical study of biopsy specimens of the colonic mucosa. Patients with ulcerative colitis were divided into groups depending on the severity of clinical symptoms, duration of disease, localization of the inflammatory process and its endoscopic activity. Proliferative activity of the cells was determined by the proliferative index of Ki-67 as follows:

where X - the number of nuclei in the field of view of the microscope. Counting was carried out in at least 10 fields of view.
Apoptosis was assessed by the expression of p53 protein in the surface and glandular epithelium of the colon.
The statistical processing of the data, after the test for equality of dispersions and distribution normality has proved that the sample does not meet the normal distribution rule, that is why the non-parametric tests have been used for groups comparison. To describe the quantitative signs we used the median, upper and lower quartiles.
Results and Discussion. In healthy individuals the PI of Ki-67 was 54 (46, 67), indicating a high proliferative activity of the cells of the colon (Table 1).
Table 1. Proliferation index Ki-67 of epithelial cells of the colonic mucosa of healthy people and patients with ulcerative colitis.
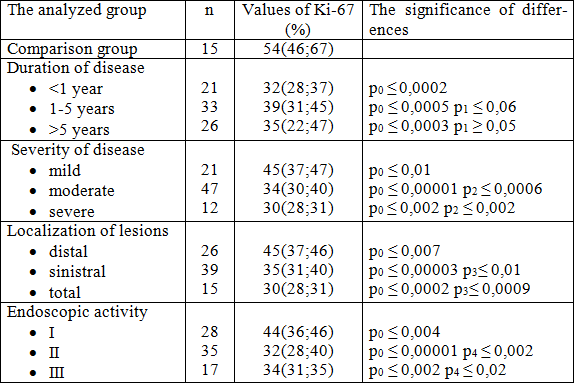
Note: p0 - Significant difference from the control group, p1 - Significant difference with disease duration of less than 1 year, p2 - Significant difference with mild disease, p3 - Significant difference with distal localization of disease, p4 - Significant difference with I degree of endoscopic activity.
As shown in Table 1, the increase of PI Ki-67 in patients with disease duration of 1 year to 5 years, compared with group 1 (p ≤ 0,06), can be attributed to increased cell proliferation, reflecting the repair processes in the colonic mucosa as a response to the therapy. With increasing duration of disease the cells' ability to proliferate decreases, as evidenced by low PI Ki-67 in the third group of patients. Depending on the severity of clinical manifestations of ulcerative colitis, we also revealed a change of PI Ki-67 (Table 1). PI Ki-67 decreased in patients with moderate (p ≤ 0,0006) and severe forms of the disease (p ≤ 0,002), compared to the patients with mild course, there was also a difference between healthy subjects and patients with ulcerative colitis of any severity stage ( p ≤ 0,05).
The analysis of the dependence of PI Ki-67 on the localization of the inflammatory process proves a significant decrease in expression of the marker in patients with ulcerative colitis in the group with the total lesion of the large intestine: 30 (28, 31), whereas in case of distal colitis PI was 45 (37, 46) (p ≤ 0, 0009).
The Table 1 also shows that an increase in disease activity is leading to PI Ki-67 decrease. There are significant differences between patients with I and II (p ≤ 0,002), I and III (p ≤ 0,02) degrees of activity.
The expression of p53 was not observed in any case in the epithelium of the mucosa of the colon in healthy people. The results of the expression of this protein in patients with ulcerative colitis are presented in Table 2.
Table 2. Expression of p53 protein in patients with ulcerative colitis.
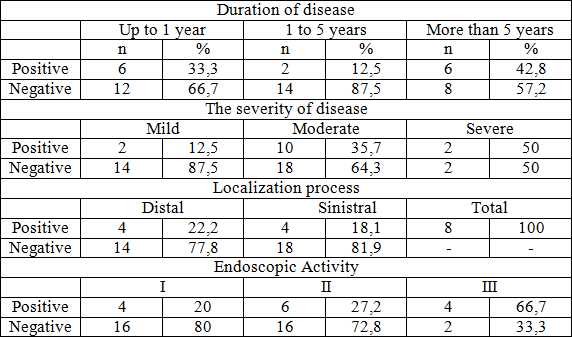
While studying the p53 indicator dependence on the duration of the disease, we have not found the patterns of expression of this protein, but there are differences in the expression of this marker between healthy subjects and a group of patients with a disease duration of 1 year (p ≤ 0,05) and more than 5 years (p ≤ 0,03).
The expression of p53 marker depended on the disease severity (12.5% - mild, 35.7% - moderate and 50% - severe). Statistically significant differences were obtained between groups of healthy subjects and patients with ulcerative colitis with moderate and severe forms of disease (P ≤ 0,03). In patients with distal colitis the positive result was obtained only in 22.2% of cases, with sinistral - in 18.1% of patients. In case of the total bowels lesion the expression of p53 was found in 100% of cases (p ≤ 0,03 compared to distal colitis).
It should be noted that the expression was most intense in areas with signs of epithelial metaplasia. We also found the dependence on endoscopic disease activity: its increase led to increased expression of p53 (p ≤ 0,05).
Conclusions:
1. In case of ulcerative colitis in acute phase the proliferative activity of the epithelial cells of the mucous membrane of the colon is significantly reduced, which is proved by the reduction of PI Ki-67 compared to healthy people.
2. The increased expression of p53 protein in the colonic mucosa in UC patients is observed, compared with the mucosa without inflammatory changes.
3. If the duration of ulcerative colitis is less than 1 year, the lowest PI Ki-67 is registered.
4. The increase of clinical and endoscopic activity of ulcerative colitis , as well as the prolonged duration of the pathological process are followed by reduced colon cell proliferative activity of the epithelium of the colon and increased apoptosis activation parameters.